What is an Oxygen-Enriched Atmosphere?
The atmospheric air we breathe is approximately 21% oxygen. Many applications use pure 100% oxygen, but others use mixtures somewhere between 21% and 100%. Industry considers oxygen concentrations above a certain percentage to be an oxygen-enriched atmosphere (OEA), but exactly what is that benchmark?
The definition of an oxygen-enriched atmosphere varies across industries, standards, guides, and codes based on varying hazard considerations within each sector. Common definitions of an OEA vary between 21%, 23.5%, and 25%; however, some industry considerations allow concentrations up to 40% or more before OEA conditions are considered. Some standards also incorporate overall pressure or partial pressure as a factor in their definition.
It’s important to understand the definition (and differences) of oxygen enrichment so that system designers and end users can understand the hazards that may be present. OEAs pose many of the same fire risks as pure oxygen. As oxygen concentration increases, materials become easier to ignite and burn more vigorously.

A Wide Range of Definitions for OEAs
The following list provides references to many of the authoritative standards available addressing OEA considerations in different industries:
| OEA Definition | Organization | Reference |
| 21% | National Fire Protection Association (NFPA) | NFPA 53, “Recommended Practice on Materials, Equipment, and Systems Used in Oxygen-Enriched Atmospheres” addresses general OEA hazards.* Other NFPA Standards, such as NFPA 99, “Health and Healthcare Facilities Code” specify a higher OEA threshold (see below). |
| 22% | The Occupational Safety & Health Administration (OSHA) | OSHA 29 CFR 1915.11 treats OEA conditions as those with equal to or greater than 22% oxygen by volume for confined and enclosed spaces and other dangerous atmospheres in shipyard employment.* (See below for additional OSHA mentions of OEA.) |
| 23.5% | The Compressed Gas Association (CGA) | CGA G-4.1, 2009, “Cleaning Equipment for Oxygen Service” (Section 1, Note 2); CGA P-39 (2015), “Guidelines for Oxygen-Rich Atmospheres;” GA P-45-2018, “Fire Hazards of Oxygen and Oxygen-Enriched Atmospheres;” and CGA PS-13-2007, “Definition of a Threshold Oxygen-Mixture Concentration Requiring Special Cleaning of Equipment define OEA as 23.5% oxygen by volume at sea level.* |
| 23.5% | The European Industrial Gases Association (EIGA) | EIGA IGC Doc 33/18, “Cleaning of equipment for oxygen service,” and EIGA IGC Doc 04/18 (Revision of Doc 04/09), “Fire Hazards of Oxygen and Oxygen-Enriched Atmospheres.” define OEA as 23.5% by volume at sea level.* |
| 23.5% | The International Organization for Standardization (ISO) | ISO 10156:2017, “Gas Cylinders – Gases and gas mixtures – Determination of fire potential and oxidizing ability for the selection of cylinder valve outlets.” defines OEA as equal to or greater than 23.5% oxygen by volume.* |
| 23.5% | National Fire Protection Association (NFPA) | NFPA 99 (2018), “Health and Healthcare Facilities Code;” and NFPA 99B (2018), “Standard for Hypobaric Facilities.” define OEA as an atmosphere in which concentration of oxygen exceeds 23.5% by volume.* |
| 23.5% | The Occupational Safety & Health Administration (OSHA) | OSHA 29 CFR 1910.146 further defines OEA conditions as those with greater than 23.5% oxygen by volume for permit-required confined spaces.* |
| 25% | The International Marine Contractors Association (IMCA) | IMCA D 031 – May 2003, “Cleaning for Oxygen Service: Setting up Facilities and Procedures;” and IMCA D 048 – January 2017, “Guidance on Surface Supplied Diving Operations Using Nitrox.” define OEA as greater than 25% oxygen by volume.* |
| 25% | The United States Military | MIL-STD-1300D(SH) June 2007, “Precision Cleaning and Testing of Shipboard Oxygen, Helium, Helium-Oxygen, Nitrogen, and Hydrogen Systems.” treats OEA conditions as those greater than 25% oxygen by volume.* |
| 25% | ASTM International | ASTM G-126 (2016): “Terminology Related to the Compatibility and Sensitivity of Materials in Oxygen-Enriched Atmospheres” defines an OEA as a fluid mixture containing more than 25-mole percent oxygen.* |
| 40% | National Oceanic and Atmospheric Administration (NOAA) | The NOAA Diving Manual treats OEA conditions as those with greater than 40% oxygen by volume.* |
| 40% | The Occupational Safety & Health Administration (OSHA) | OSHA 29 CFR 1910.430 (i)(2) defines OEA conditions as those with greater than 40% oxygen by volume for commercial diving equipment/operation except umbilicals.* |
| 50% | Association of Diving Contractors International (ADCI) | Consensus Standard 6 defines OEA for hose assemblies at greater than 50%.* |
*Note these standards DO NOT explicitly state that environments with lesser O2 concentrations are always “safe” or do not need to be cleaned.
It is noteworthy that the historical rationale for each standard’s definition for OEA may differ, and not all of them are concerned with fire hazards. For example, the standards that define an OEA between 21% and 25% site rationale including air quality and the upper concentration of oxygen for compressed air blending, respiratory concerns for human consumption, as well as concerns for fire, flame spread, and burning rate of flammable materials.

Oxygen-enriched Atmospheres for Diving
As you can see from the standards above, most industry guidelines consider an OEA to begin between 21-25% oxygen. However, the diving industry has historically been a notable outlier in defining OEAs, considering the threshold to begin at 40% oxygen or even higher.
Divers sometimes use a mixture of nitrogen and oxygen known as “nitrox” in which oxygen concentrations are typically 32% or higher. When diving, water pressure causes nitrogen to dissolve into the bloodstream. When divers surface from greater depths, they must do so gradually to avoid dangerous decompression sickness (also known as “the bends”). With nitrox, the increased concentration of oxygen (less nitrogen) allows divers to dive deeper and for longer durations.
The application of these OEA guidelines in the diving industry can be problematic because often users misinterpret the standard and assume that anything less than 40% oxygen can theoretically be used with no modifications to breathing equipment or special cleaning required.
It’s important to note that while recreational diving standards mention that cleaning is required for OEAs above 40%, they DO NOT explicitly state that anything below 40% is “safe.” The question becomes: at what oxygen concentration should special care and cleaning be applied?
WHA engineers have been addressing this question for many years, including a notable article and presentation regarding scuba oxygen applications to the Divers Alert Network in 2000. OEA questions continue to be received from various industries, including industrial gas plants (when encountering a leaking oxygen pipeline), high-pressure air compressors, and liquid oxygen transfilling operations.

OEAs pose potential fire risks at any concentration
Although lower-concentration OEAs may not behave exactly like pure oxygen, they still exhibit very real oxygen fire risks. All materials will ignite and burn more easily in the presence of elevated oxygen concentrations, and nonmetals are particularly susceptible to fire risks with even slightly elevated oxygen.
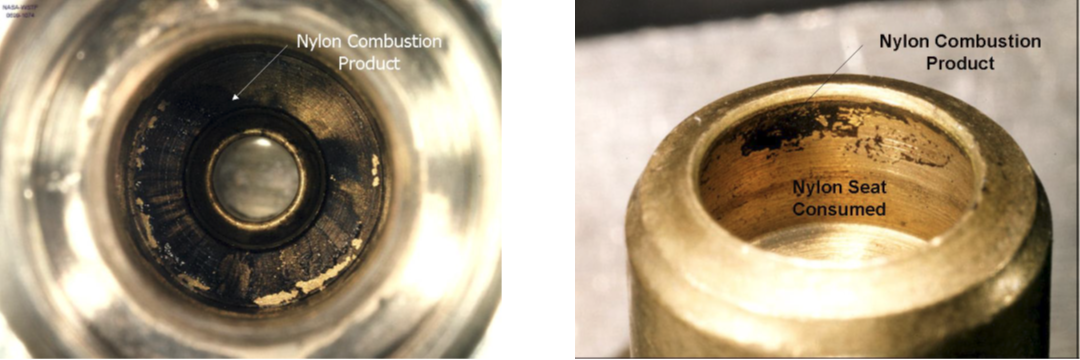
For example, at least two fires involving cylinder valve seats in 46% nitrox scuba systems were investigated at NASA’s Neutral Buoyancy Lab (NBL) (pictured above). Below, you can see artifacts from one of the incidents, including the combustion remains of the nylon seat. Nylon is flammable under these conditions and similar fires have been reported in cylinder valves using even standard compressed breathing air.
Takeaway #1: WHA generally recommends that special care be taken where oxygen concentrations exceed 21%, including consideration for cleanliness to remove flammable contaminants. Further, WHA would encourage users to follow the guidance specified in the standards most relevant to their industry and application.
Though nitrox fires may be less frequent than those involving pure oxygen, many have still been reported. For example, in nitrox fill stations, fire incidents in systems using less than 40% oxygen are known to have occurred in aluminum and carbon steel compressor blocks, aluminum-bodied filter towers, fill station panel valves and regulators, and some pre-compression nitrox generating equipment used for continuous blending. The cause of these fires varies, but many are related to the ignition of hydrocarbon or particulate contamination, be it from poor maintenance or otherwise, but that may have been avoided through proper cleaning.
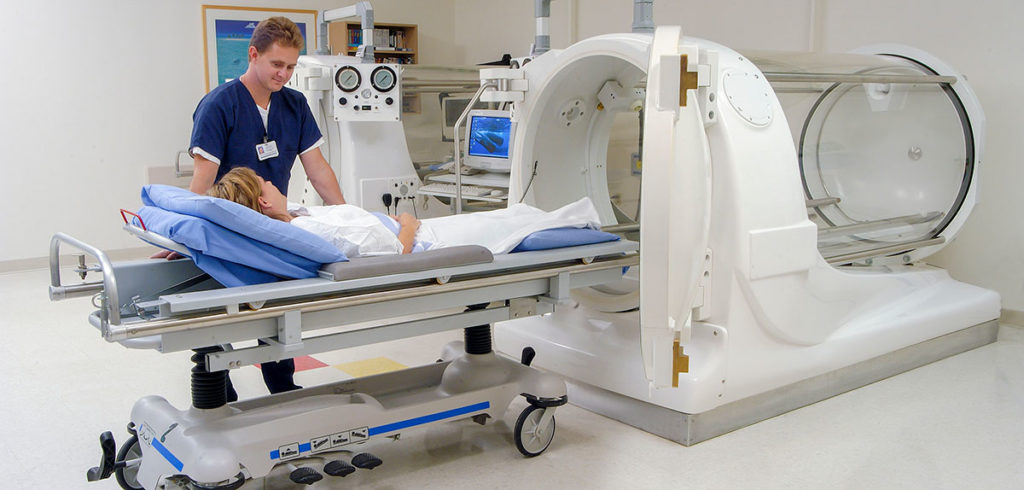
Hyperbaric oxygen chambers provide an example of systems that have experienced fires even at low oxygen pressures but high oxygen concentrations. One of the most tragic chamber fires occurred in a 1.8-atmospheres multi-place air chamber in Milan, Italy, in 1997, killing 11 people.
WHA Recommends Oxygen Hazard Analysis for any OEA

It’s important to remember that oxygen concentration is only one factor in a much larger set of conditions considered in a typical oxygen hazard analysis. Fire risk is also influenced by other factors, including oxygen pressure and temperature, materials of construction, and the equipment’s function.
Consider the fire triangle illustration. Many factors can influence the severity of the oxidizer (concentration, pressure, thickness, flow, etc.). When these factors increase, the other sides of the triangle can also become more severe as materials become more flammable and easier to ignite. Thus, all three sides of the triangle affect overall fire risk.
It can be problematic to simply draw a “line in the sand” when defining an OEA and assume this establishes a “safe” operating environment. Such an assumption can create a false sense of security if system designers and end users fail to consider other factors that increase the probability and consequence of ignition.
Takeaway #2: WHA advocates that an oxygen hazards analysis be performed when needed to assess the specific fire risk associated with contaminants in any system with enriched oxygen. Acceptable fire risk is determined not just by concentration, but other factors as well.
A Final Word on Oxygen-Enriched Atmospheres
So then, what exactly defines an oxygen-enriched atmosphere? At WHA, we consider any oxygen concentrations above 21% to be oxygen-enriched, and as such it may require special considerations such as cleaning to minimize the risk of ignition and fire. However, WHA also encourages users to follow the guidance specified in the standards most relevant to their industry and application. Keep in mind that an oxygen hazards analysis can be used to analyze the specific fire risk associated with contaminants in any OEA because an acceptable fire risk is determined not just by concentration, but other factors as well.
If you have safety questions about oxygen-enriched atmospheres or need to train your staff, don’t hesitate to reach out to us at WHA. Our experienced engineers can equip you with oxygen hazard analysis, technical training, precision cleaning, and other oxygen safety services.
Acknowledgements: WHA would like to thank Richard Barry (VP of Hyperbaric Services at Healogics, Technical Committee Chair for NFPA 53) for his invaluable contributions to this article.
Share this entry
Related Articles
Nonmetals Oxygen Compatibility Requirements: Guide to CGA/EIGA Compliance
The Compressed Gas Association (CGA) and European Industrial Gases Association (EIGA) have harmonized key documents providing guidance…
WHA Launches Oxygen Equipment Supplier Certification Program
Ignition and combustion hazards are present in almost all oxygen and oxygen-enriched systems, and catastrophic fires have…
Oxygen Cleaning Service Team Receives Vendor Award
WHA’s oxygen cleaning service team recently received recognition from its client, Trace-A-Matic, for excellent service in a…
Request an expert
consultation
Contact us to request a free consultation with an experienced engineer who can help you better understand your needs and our solutions.



