Case Study: Large industrial ball valve fire
Case Study: Large industrial ball valve fire
In 2001, WHA International, Inc. investigated a major industrial oxygen fire that resulted in significant damage as well as personnel injury and one death. The fire was initiated in a 6 in. (150 mm) ball valve while it was being opened under a low-pressure differential, which caused extensive burnout. The results of this incident emphasize the extreme nature of fires in oxygen pipeline systems and underscore several important considerations in the design and operation of these systems.
A tragic oxygen fire occurs
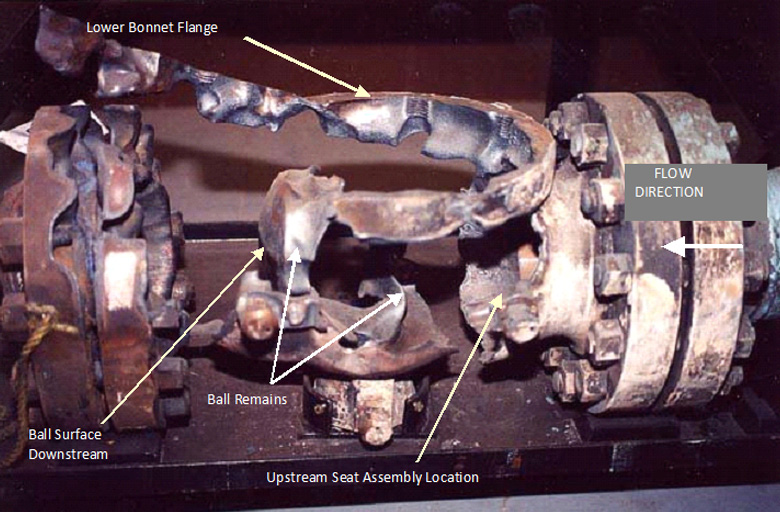
In July of 2001, a fire occurred in a 6 in. (150 mm) ball valve being used as an oxygen pipeline isolation valve (shown here).
Just prior to the fire, the valve had been closed and a leak check was performed on the valve by bleeding off pressure downstream and monitoring the pressure differential across the valve. During this leak check, system data indicated that the upstream pressure was approximately 550 psig (3.8 MPa) and the downstream pressure was approximately 510 psig (3.5 MPa), or roughly a 40-psig (0.28 MPa) differential pressure.
At this point, the valve was to be re-opened to establish flow and provide full system pressure. As the valve was being opened manually by the hand wheel, ignition and fire developed within the valve that consumed most of the valve internals and surrounding valve body, as well as downstream piping and flanges. Tragically, the operator was killed in the incident.
Looking to WHA for answers
WHA International, Inc was contacted to provide forensic investigation and failure analysis of the incident. WHA’s unique industry experience and expertise were exactly what the client needed to determine the origin and cause of the fire. Thorough understanding of the accident was critical to preventing such a tragic incident from occurring again.
WHA’s approach to investigating oxygen fires is detailed and thorough. Our engineers apply the concepts from international standards that they themselves helped develop, including ASTM G 88 (Standard Guide for Designing Systems for Oxygen Service), G 63 (Standard Guide for Evaluating Nonmetallic Materials for Oxygen Service), G94 (Standard Guide for Evaluating Metals for Oxygen Service) and G-145 (Standard Guide for Studying Fire Incidents in Oxygen System).
Evidence reconstruction
WHA’s forensic engineers first began by documenting and reconstructing the available evidence and examining a similar “exemplar” valve that was also installed in the same oxygen system. This undamaged exemplar valve proved useful to the investigation in many ways, providing a reference for the valve’s actuator positioning and samples of materials, especially lubricants, which had largely been consumed in the incident valve. The exemplar valve was referenced, along with the use of other analytical tools, to reconstruct the burned fragments into their approximate original positions and to create a three dimensional model of the valve’s internal flow passages.
The incident reconstruction provided three major insights:
- No burning or melt patterns were observed upstream of the main seat assembly, compared to significant burning and evidence of melt-flow extending downstream from this location. These burn patterns suggested an early local origin in the proximity of the upstream seat. This observation is consistent with previous experience indicating that for flowing oxygen systems of primarily metallic components, burn patterns usually do not extend upstream of the local origin of the fire. Propagation typically progresses in a “fire follows flow” pattern and generally begins to quench once the pressure containment is breached and pressure is lost.
- Burning on the valve body was heaviest on the back and downstream side of the valve. The front side of the body also sustained significant damage as only discontinuous segments remained, mostly under the ball and associated with the lower shaft housing. The upper shaft and top of the ball were completely consumed. Only the upper bonnet flange remained intact (connected to the actuator, as shown in Figure 3 below), but it was heavily lanced during the event.
- The recovered ball fragment shows a strong preference for burning from upstream to downstream. Because of the significant burning upstream of the ball element and on the upstream face of the ball, the evidence indicated that the fire’s local origin was upstream of the ball element.
Ignition Mechanisms and Flow Analysis
The fire damage and melt/flow patterns discussed above suggested that ignition took place within the upstream seat retainer, which contained a wave spring packed with a lubricating grease as supplied through two grease-injection ports in the valve body. Samples of this lubricating grease were taken from the exemplar valve and analyzed to be consistent with a heavy hydrocarbon grease, which is highly flammable and easy to ignite in oxygen. This same lubricant is believed to have also been within the incident valve at the time of the fire. This evidence suggested that contaminant promoted ignition could have occurred while the valve was opening due to the mechanical energy created by the pressure differential causing high-frequency movement of the contaminated wave springs and the re-establishment of flow through the valve.
However, conditions at the time of the fire gave credibility to another potential ignition source as well. Though the valve was being opened with a low differential pressure, initial calculations using standard isentropic flow equations for compressible fluids estimated the gas velocities through the valve to be sufficient to potentially ignite particles. Because of this, WHA engineers also considered particle impact ignition as a possible ignition source.
“It’s important to remember that just because a component has been in operation in oxygen for a long time, it does not guarantee that it’s safe. This valve had been in operation for several years before the conditions were met to cause an incident.”
Elliot Forsyth, WHA International, Inc.
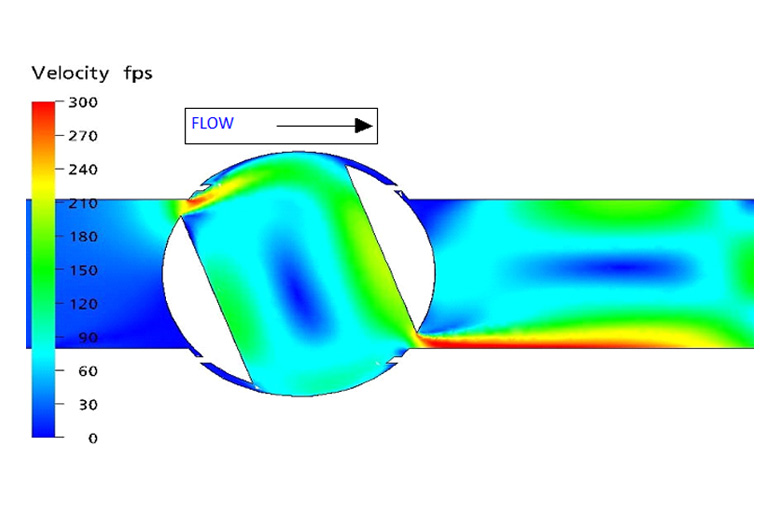
To further evaluate the most probable ignition scenario and associate the observed melt/flow patterns, a 3D model of the valve was developed to approximate the geometric relationships of the valve internals and perform computational fluid dynamics (CFD) modeling of the flow conditions believed to be present in the valve at the time of the fire. Of specific interest were gas velocities and vector streamlines, as well as pressure and temperature conditions throughout the valve. The WHA CFD analysis predicted that the gas velocity would have exceeded 300 ft/s (90 m/s) immediately downstream of the valve’s seat, both where the flow entered the ball’s interstage (upstream) and where flow exited the interstage (downstream). The CFD analysis also predicted very turbulent flow downstream of the valve.
The characteristic elements for particle impact ignition include the presence of particles, high gas velocities, impingement locations, and flammable materials. Test data has shown that gas velocities greater than approximately 150 ft/s (45 m/s) are capable of supporting particle ignition in oxygen. Further, design guides for oxygen systems recommend limiting gas velocities to below 100 ft/s (30 m/s) to minimize particle impact ignition. The CFD analysis predicted that flow velocities in excess of these thresholds were developed as the flow was sweeping past the upstream seat retainer and leading edge of the ball element.
Conclusions and Lessons Learned
Despite the fact that the CFD model predicted that all characteristic elements for particle impact ignition could have been present at the time of the fire, WHA’s analysis of the burn patterns and post-fire evidence indicated that the most probable cause of this fire was contaminant promoted ignition. The CFD analysis provided key insight into each of these ignition mechanisms and also confirmed the relevant probable gas pressures and velocities inside the valve.
The first take away from this investigation is the importance of minimizing hydrocarbon-based contaminants in oxygen systems. Hydrocarbon contaminants require relatively little energy to ignite yet burn vigorously in oxygen with energies capable of propagating a fire to other materials (especially if gross quantities are present, as was the case in this valve).
REMEMBER: Always use approved lubricants in oxygen systems. All oxygen components should undergo thorough oxygen cleaning, and personnel should be properly training in maintaining cleanliness through proper installation and maintenance practices.
A second lesson learned in this analysis is the importance of proper operation of isolation valves in oxygen. These valves are typically purposed only for isolating pressure and flow, not for throttling or controlling flow. As such they are generally to be operated fully open or fully closed, and not operated under pressure differentials which can produce very high gas velocity through the valve. A “zero pressure, zero flow” operating philosophy often applies to isolation valves in oxygen, where the valve is pre-positioned open before pressurized (zero pressure) or where pressure is equalized across the valve before opening (zero flow). If an isolation valve in an industrial oxygen system is not designed to be operated under pressure differentials (during system start-up or re-start for example), often a small-diameter bypass valve is used to slowly equalize pressure across that valve and enable safe operation.
“In some circumstances it is not well appreciated that even a 7% pressure differential (93% equalized), as was the case in this fire, can produce gas velocities that are significantly greater than the preferred maximum velocities to avoid particle impact ignition.”
Dr. Barry Newton, WHA International, Inc.
REMEMBER: Safe operating procedures and best practices should be clearly outlined. All personnel involved in the operation of an oxygen system should receive proper technical training. All oxygen systems should be thoroughly analyzed for oxygen hazards, including the potential for mis-operation or failures that could lead to fires.
Share this entry
Related Articles
WHA Launches Oxygen Equipment Supplier Certification Program
Ignition and combustion hazards are present in almost all oxygen and oxygen-enriched systems, and catastrophic fires have…
Oxygen Cleaning Service Team Receives Vendor Award
WHA’s oxygen cleaning service team recently received recognition from its client, Trace-A-Matic, for excellent service in a…
Case Study: Fire Station Medical Oxygen Cylinder Fire
A medical oxygen cylinder experienced a catastrophic failure and burnout during a routine fire station equipment check
Request an expert
consultation
Contact us to request a free consultation with an experienced engineer who can help you better understand your needs and our solutions.