Where Does Hydrogen Come From? Hydrogen Production Methods
Hydrogen’s unique properties make it an excellent fuel for industrial processes, hydrogen fuel cells, and other clean energy applications. It’s the most abundant element in the universe, yet its pure molecular form (H2) is rare on Earth. Any industry using hydrogen must produce or generate H2 by separating it from other elements in molecules like water (H2O) or hydrocarbon fuels.
As hydrogen use increases, scientists and businesses are exploring cheaper — and greener — ways to generate hydrogen. The world used over 90 million tonnes in 2020, with projections to increase by more than 30% in 2030.[1] Many countries aim to reach a carbon-neutral, or even carbon-negative, planet by 2050, and hydrogen can play a vital role in this energy revolution.
So, where does hydrogen come from, and how clean is it? We spoke with several of WHA’s hydrogen experts to get the inside scoop on hydrogen production.
How clean is hydrogen energy?
When hydrogen burns, it simply produces water vapor – and nothing else. This considerable benefit has led many to brand hydrogen as “clean” or “green” energy, but is it always good for the environment?
“It’s important to remember that hydrogen has always been classified as an energy currency,” explains Dr. Beeson, principal chemist and resident hydrogen expert at WHA. “It’s not necessarily a resource; it’s a carrier of energy – easily transported or stored.”
Therefore, hydrogen’s environmental impact depends on how it is produced. It can be made using environmentally friendly methods, but the world still relies almost entirely on fossil fuels to create hydrogen. In fact, hydrogen production was responsible for nearly 900 million tonnes of direct CO2 emissions in 2020.[1]
New hydrogen production methods show promise, but many still create some CO2 or other harmful greenhouse gases. Each production method is different, with its own pros and cons when it comes to environmental impact.
These complex environmental considerations have led to what some call the hydrogen rainbow. Because hydrogen production methods may not be 100% green, innovators are calling them by other colors.
- Green hydrogen: produced with no harmful carbon emissions
- Blue hydrogen: produced from natural gas paired with carbon capture technologies used to limit carbon emissions
- Grey hydrogen: produced from natural gas without carbon capture technologies
- Black/brown hydrogen: produced from coal and other fossil fuels
- Pink hydrogen: produced via electrolysis powered by nuclear energy
- Yellow hydrogen: produced via electrolysis powered by solar energy
- Turquoise hydrogen: produced by a methane pyrolysis process
- Gold hydrogen: biohydrogen produced by oil-eating microbes
The rainbow of developing hydrogen technologies can be a lot to keep up with, and only time will tell which methods will secure a foothold amidst fast-paced industry growth.
Established fossil-based hydrogen production methods
Today, the hydrogen economy primarily relies on a few tried-and-true production processes. In 2020, 79% of the world’s hydrogen was created from fossil fuels at dedicated production plants. The remaining 21% was created as a byproduct at refineries and facilities designed to create other products.[1]

Common fossil fuel-based hydrogen production methods include:
- Steam methane reformation: It uses high-temperature pressurized steam to produce hydrogen from natural gas (fuels like ethanol, propane, or gasoline may also be used). First, methane reacts in the presence of a catalyst to produce hydrogen, carbon monoxide, and carbon dioxide. Then, in a secondary”water-gas shift reaction,” the carbon monoxide and steam react again to produce carbon dioxide and more hydrogen. A final “pressure-swing adsorption” process then removes carbon dioxide and other impurities.[2] Steam methane reformation accounts for the majority (60%) of global hydrogen production today.[1]
- Coal Gasification: This process uses high-temperature steam and oxygen to break down coal into a gaseous mixture of hydrogen, carbon monoxide, and carbon dioxide. This mixture is then further refined using a water-gas shift reaction and purified with a pressure-swing absorption process.[3] Coal refining accounts for 19% of global hydrogen production today.[1]
- Partial Oxidation: Heavier hydrocarbons are not suitable for steam reforming until they have been broken down by a method like partial oxidation. In this process, refineries partially combust a fuel-air or fuel-oxygen mixture. This produces syngas with hydrogen, carbon monoxide, and carbon dioxide. This mixture is then further refined using a water-gas shift reaction and purified with a pressure-swing absorption process.[4]
Water electrolysis methods
Electrolysis uses electricity to break water molecules (H2O) into pure hydrogen and oxygen. When powered by renewable energy sources, electrolysis can theoretically create 100% green hydrogen.
Electrolysis systems supply power between two electrodes (a cathode and anode) to drive the process of dissociation of water into its oxygen and hydrogen components. There are four main electrolysis technologies that all work around this basic concept: alkaline; proton exchange membrane (PEM); solid oxide electrolysis cell (SOEC); and anion exchange membrane (AEM).
With demand for hydrogen expected to reach 735 million tonnes (808.5 million tons) by 2050, the race is on to bring more electrolyzers online and further develop the technologies. Industry analysts predict that manufacturers will reach 100GW of capacity per year by 2030, dropping the price of hydrogen from $4/kg to $1.50/kg.[1]
As demand rises, innovators are also finding ways to improve the electrolysis process.
- Developing cheaper materials: Scientists in Australia have developed a method to reduce the cost of generating hydrogen through alkaline electrolysis. They’ve replaced ruthenium, platinum, and iridium with nickel and iron. While these two metals aren’t suitable catalysts on their own, combining them at the nanoscale gives this combo high efficiency. At current prices, iron and nickel are thousands of times cheaper than ruthenium, iridium, and platinum, which means this process can be much more cost-effective without sacrificing production.[5]
- Scaling up electrolysis systems: Most systems have only until recently been built on the MW energy scale, but to meet future demand, they will need to scale to the 100 MW scale, GW scale or larger. There are ongoing efforts to demonstrate the viability of electrolysis at the GW scale, and one study suggests that larger production facilities could cut costs by 40% in the short term and up to 80% in the long term.[6]
- Saltwater electrolysis: Researchers at Stanford University have developed a process that uses seawater to produce hydrogen. Negatively charged chloride molecules in seawater salt can corrode the anode in traditional electrolysis systems, limiting their lifespan and effectiveness. A typical electrolysis anode only lasts about 12 hours in saltwater, but the Stanford process extends the anode’s life to over 1,000 hours. It can also run up to 10 times more electricity through the device.[7]
Biohydrogen production methods
Several new “biohydrogen” processes feature microorganisms, algae, or chlorophyll to create hydrogen. These methods utilize fermentation, photosynthesis, and other natural biological mechanisms. Biohydrogen shows broad potential because of the ready availability of organic waste worldwide.
However, biohydrogen production can be relatively complex, and large-scale optimization is yet to be proven. Only time will tell if these innovative methods can achieve cost parity with other established means of production.
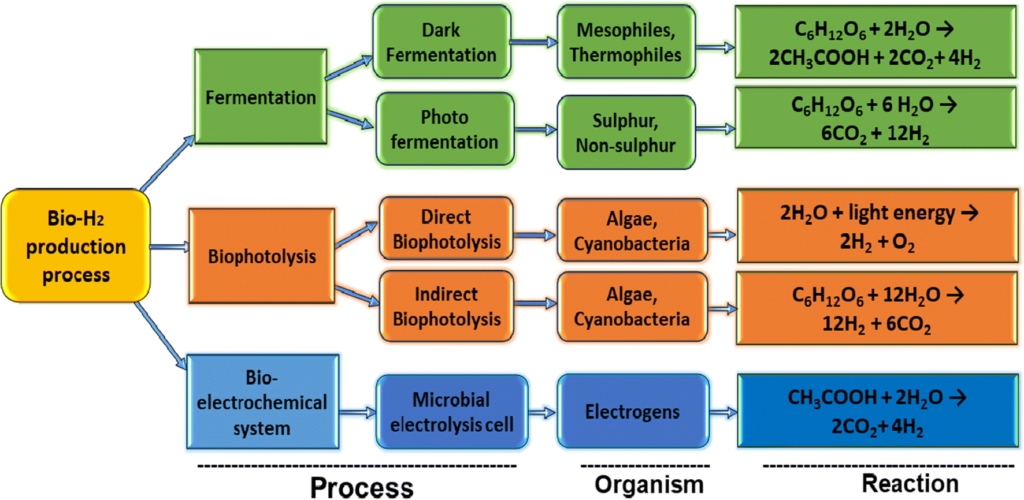
Severalcompanies like Cemvita have even discovered bacteria that eat oil and release hydrogen and carbon dioxide as waste gases. These microbes can produce hydrogen from depleted oil wells that are no longer economically viable.
Other novel hydrogen production methods
With the rising demand for hydrogen and green energy solutions, many innovators are developing entirely new production methods.
A few novel production methods include:
- Plasma-assisted gasification: Companies like Boson Energy and SG H2 Energy (SGH2) use a plasma-assisted gasification process to convert non-recyclable waste into hydrogen, carbon dioxide, and a molten slurry that cools down into a glass-like rock. One new plant Located in Lancaster, California, SGH2’s plant is projected to process 40,000 tons (36,363 tonnes) of waste to produce 3.8 million kilograms (4,180 tons) of hydrogen a year.
- Photocatalytic water splitting: Water dissociates into hydrogen and oxygen through the natural process of photosynthesis. Ongoing studies are exploring methods to replicate this reaction at scale using only water, photons (light energy), and a catalyst.[8]
Carbon capture technologies
Many hydrogen production methods, like fossil fuel refining, still produce carbon dioxide, but what if that carbon could be contained or recaptured? Some companies are exploring carbon capture, utilization, and storage (CCUS) to offset some of the environmental impacts of their processes.
This concept becomes even more interesting when combined with using hydrogen to create synthetic fuels. Refining processes can be used to break fuels down, but they can also build them back up. It’s possible to combine hydrogen with carbon to create synthetic fuels equivalent to or identical to the fossil fuels we already use. The US military has even recently created synthetic JP8 jet fuel.
If companies could capture and combine atmospheric carbon with hydrogen, they could turn that back into fuel. This could theoretically facilitate a closed-loop, net-zero system in which no new carbon enters the atmosphere.

Pursuing “Energy Diversification”
It’s easy to get excited when new hydrogen technologies make headlines – “Is this the answer? Will it solve all our energy problems?”
Cory Kreutzer, mechanical engineer, chemist, and resident hydrogen expert, explains that emerging hydrogen technologies are as much about energy diversification as they are about energy revolution: “Our energy needs have become so complex that no one technology is enough to take care of everything. Every energy source has its strengths and weaknesses, and we can’t rely on one alone … Hydrogen is beneficial because it integrates well with all sorts of existing technologies. Fuel cells can work on a massive industrial scale or as small portable energy solutions. They help diversify our options.”
How does hydrogen help provide energy diversity? Its unique chemical properties offer a myriad of promising solutions:
- Lightweight energy storage: Hydrogen fuel cells are much lighter than chemical batteries. They’re good candidates for planes and spacecraft if coupled with lightweight hydrogen storage solutions.
- High-capacity energy storage: Because of their weight-to-energy ratio, hydrogen also provides better energy storage solutions for heavy vehicles like ships, submarines, trucks, buses, and trains. These heavy-duty hydrogen systems can also be refueled in minutes to hours instead of the hours to days it takes to recharge batteries.
- Electrical grid balancing: Many energy systems experience seasonal or intermittent periods of fluctuating production and demand. Some energy sources like hydroelectric dams always generate a set amount of power that will go to waste if unused. Hydrogen can help capture and store this excess power generated by wind, solar, hydroelectric, or nuclear generators. That hydrogen can be stored and fed back into the system during periods of high demand.
- Remote energy applications: Hydrogen can help provide continual energy in remote locations and third-world countries where communities don’t have access to reliable electrical grids. It can provide primary or backup power when coupled with renewable sources like solar energy. In addition, hydrogen is easily transported by road where long-distance power lines are unavailable.

How can the hydrogen economy grow safely?
WHA’s hydrogen experts explain that its chemical properties provide unique benefits and risks.
“As the hydrogen economy continues to grow, there’s a significant risk with companies and individuals assuming that hydrogen behaves similarly to natural gas or other fuels,” explains Cory. “In our technical consulting and training courses, we work with our clients to ensure they understand the unique properties of hydrogen and help them assess their systems to make sure they are considering those properties safely. Our recently-updated Hydrogen Combustion Risk Analysis (HCRA) process is specially designed to address the properties within the demands of evolving hydrogen applications.
Unfortunately, hydrogen fires and explosions can potentially disrupt the industry’s entire momentum. There have been enough past failures, fires, and explosions that H2 has a reputation to overcome. It’s our job at WHA to help the hydrogen economy continue to grow safely and securely.”
[1] https://iea.blob.core.windows.net/assets/e57fd1ee-aac7-494d-a351-f2a4024909b4/GlobalHydrogenReview2021.pdf
[2] https://www.energy.gov/eere/fuelcells/hydrogen-production-natural-gas-reforming
[3] https://www.coalage.com/features/hydrogen-from-coal/
[4] https://netl.doe.gov/research/Coal/energy-systems/gasification/gasifipedia/oxidation
[5] https://www.nature.com/articles/s41467-019-13415-8
[6] https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2020/Dec/IRENA_Green_hydrogen_cost_2020.pdf
[7] https://news.stanford.edu/2019/03/18/new-way-generate-hydrogen-fuel-seawater/
[8] https://www.nature.com/articles/s41586-020-2278-9
Share this entry
Related Articles
Hydrogen Tank Explosion Demonstration
Typically, hydrogen is safely stored as a purified gas or liquid. Although hydrogen is a fuel, it…
Hydrogen Cleaning: Ensuring Safety and Reliability in Pressurized Hydrogen Systems
Hydrogen is an increasingly important and prominent energy carrier, with applications ranging from fuel cells to electrolyzers…
Battery Safety Insights with Nic Linley, Electrical Engineer
Battery technology continues to evolve rapidly in the 21st Century, powering an expanding array of devices and…
Request an expert
consultation
Contact us to request a free consultation with an experienced engineer who can help you better understand your needs and our solutions.



